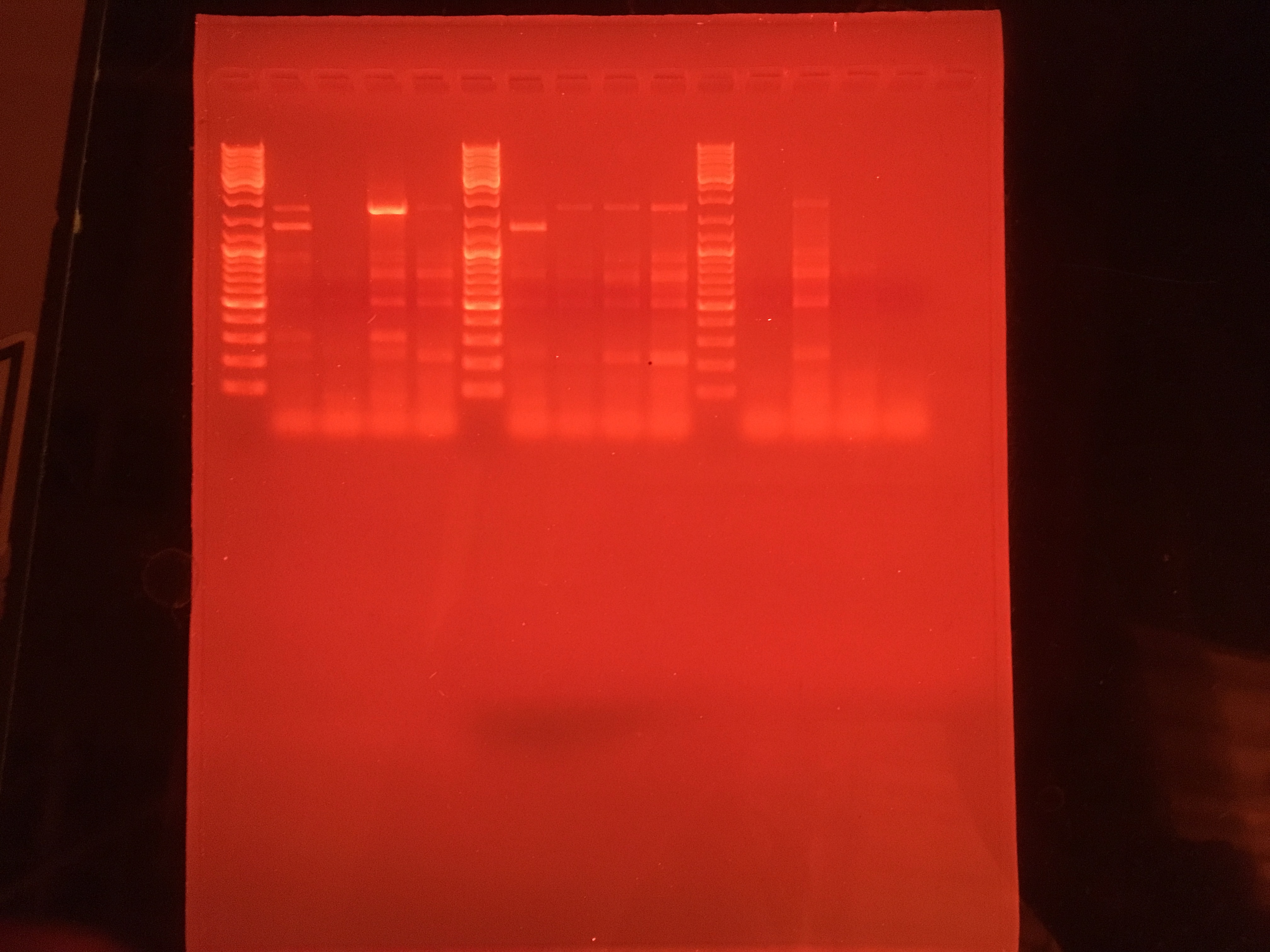
November 21, 2019 - Oyster Species Determination Using Gel, Olympia Oyster Ethanol Concentration Results, and Possible Research Project Ideas
Today, I did three main things:
- Helped Sam continue the process of discerning the species of oysters from the oyster distribution company by putting the PCR samples into a gel
- See the results from the experiment done with different ethanol concentrations on the preservation of oyster larvae samples that I helped Laura with
- Met with Dr. Roberts and Laura to discuss potential research projects for next quarter
Oyster Species Determination Using Gel
Steps for gel electrophoresis and analysis:
- The gel was made using a mix of agar and a TAE buffer solution
- The gel was placed into a container
- Container was filled with more TAE buffer solution
- Using a pipette, DNA ladder mix and the samples were placed in the following order (from left to right):
| Well# | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Solution/Sample Type | DNA Ladder Mix | 9 | 10 | 11 | 12 | DNA Ladder Mix | 21 | 22 | 23 | 24 | DNA Ladder Mix | 33 | 34 | 35 | NTC |
| Volume | 5 uL | ~25 uL | ~25 uL | ~25 uL | ~25 uL | 5 uL | ~25 uL | ~25 uL | ~25 uL | ~25 uL | 5 uL | ~25 uL | ~25 uL | ~25 uL | ~25 uL |
- Ethidium bromide was added to buffer solution outside of the gel
- A cover with black and red wires connecting to the box was put on top of the gel box
- The wires were connected to a power supply
- The power supply is set to 100 V and runs for about 1 hour
- The power supply is turned off and the gel is removed from the box and placed on top of an Ultraviolet Translinker to analyze the wells.
Here are the results from the gel:
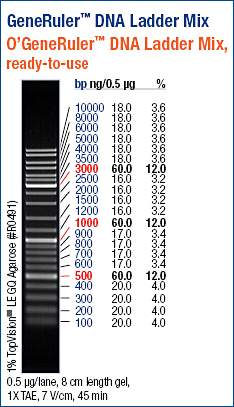
The DNA Ladder Mix has the following base pair amounts:
Olympia Oyster Ethanol Concentration Results
The different ethanol concentrations with 2 larvae samples (7 and 50) that I made last week with Laura were analyzed under a microscope today to see the quality of larvae preservation. Things that were taken into consideration when assessing quality include the “roundness” of the shells (which also means an absence of excess tissue around the shell) and the amount of surrounding debris.
Each sample was mixed carefully using a pipette before placing the liquid onto a glass slide. Then a cover slip was placed on top of the mixture to analyze under the microscope.
The results from all of the different ethanol concentrations (ranging from 70% to 95% ethanol) showed that the larvae from sample 7 were constantly in excellent condition while the larvae from sample 50 varied from “great” to “ok” condition. This is especially interesting because it seemed like sample 7 would have ended up in a poorer state since Laura noticed that the sample was softer than what is normally expected (stated in last post here). Since the preservation quality of the samples were almost constant throughout all of the different ethanol concentrations in both samples, the quality of preservation may have something to do with the samples themselves rather than the concentration of ethanol in which they are preserved.
Thus, we decided to use a 70% ethanol concentration in order to utilize the least amount of ethanol necessary to obtain the desired preservation quality.
I made a 70% ethanol mixture using a 50 mL tube, which I filled to 45 mL. Using 190 Proof ethanol, I created 45 mL of 70% ethanol by using the equation C1V1 = C2V2, and found that ~ 33 mL of ethanol and ~12 mL of water should be used to create this concentration (the volume of solutions added did not need to be extremely precise since we discovered that the concentration of the solution does not have a strong effect on preservation quality).
Possible Research Project Ideas
I spoke with Dr. Roberts and Laura about my research project interests for next quarter, and Laura has some Australian flat oyster samples that could be analyzed for gene expression relating to reproduction or heat stress.
I have decided to help in the analyzation for both reproduction and heat stress gene expression since I would like to gain a multitude of scientific and analytical skills. Once I have explored the process of analyzing both reproduction and environmental stressors, if there is a certain variable that I am more interested in, I may focus my research more towards one variable over the other.
Next Steps
- Look at the results of the gel to determine the oyster species analyzed
- Start transferring the frozen larvae samples into ethanol
- Due to the soft quality of sample 7 contrasting with the excellent condition of the samples in all ethanol concentrations, soft samples should be noted on the spreadsheet when transferring larvae to ethanol
- Read resources that Laura sent to me on the Australian flat oyster